
I now have two aluminums,Īnd so it looks like the aluminums are balanced,Īnd they are indeed balanced. I have one aluminum here, "well why don't I just double the number "of aluminums right over here?" I could just write a two in front of it, so now this has two aluminums, so I no longer have one aluminum here. To say, "Okay, if I've got "two aluminums here and So how do we do that? Well one thing might be The number of oxygens, this number and that Number should be the same, and we have to balance Of aluminums on both sides, this number and this Just have miraculously an oxygen atom appear out of nowhere.
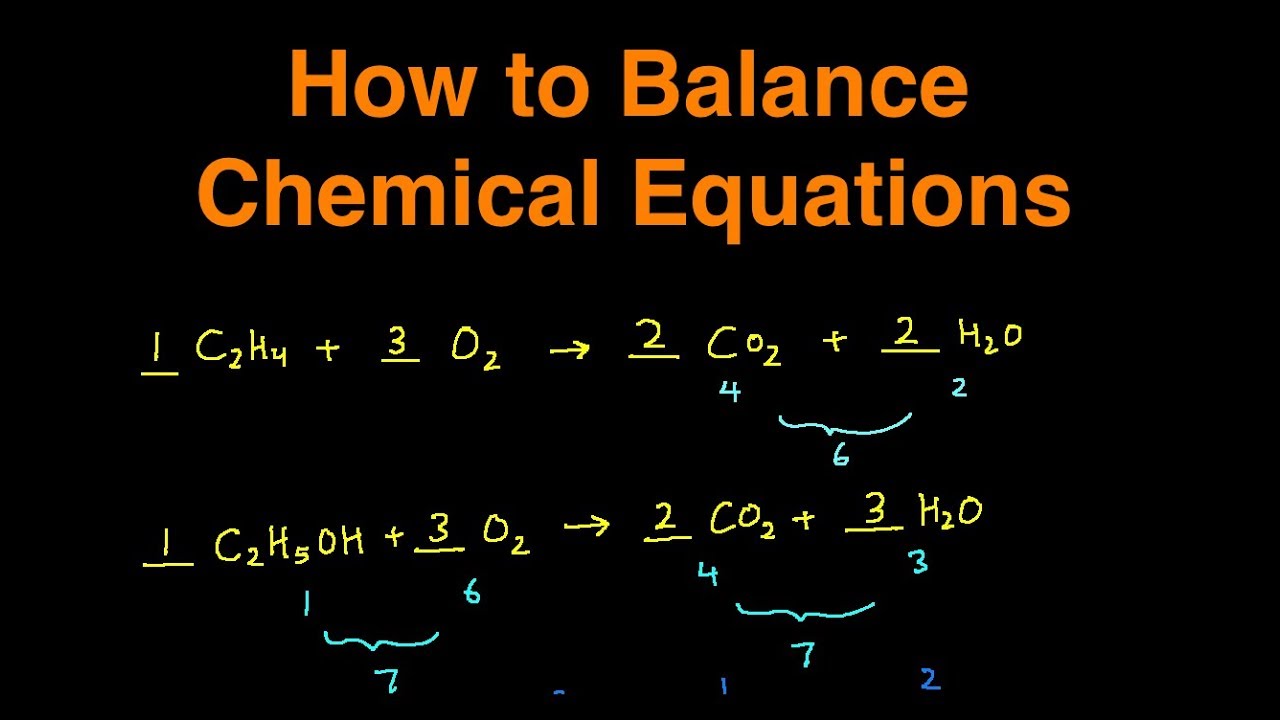
And then over here in theĪluminum oxide molecule, we have three. Of aluminums on both sides, and the same thing is And so aluminum just can'tĪppear out of thin air by virtue of some magical reaction. The right-hand side? Well on the right-hand Here on the left-hand side, how many aluminums do we have? Well on the left-hand You might notice that you don't have the same number "What do I have to balance?" Well if you look carefully, And so you might say, "Okay, well what's "the balancing business all about? "I have a chemical reaction. And the aluminum oxide molecule has two aluminum atoms and three oxygen atoms. The appropriate conditions they will react to form aluminum oxide. So if I take an atom ofĪluminum and I add it to a dioxygen molecule, so a molecule that has two oxygens with it, under Is a chemical equation? Well this is a chemicalĮquation right over here. If we work through this carefully and methodically,Īnd we also appreciate the art of balancing chemical equations, that it's actually not too bad. You can think about it this way 1 atom (Mg) + 2 compounds (HCl) combines in a reaction to form the products of 1 compound (MgCl2) + 1 Molecule (H2).Įquations is one of those concepts in chemistry that

This equation is easily balanced by placing the coefficient "2" in front of molecule (HCl) to form the balanced equation (Mg) + 2(HCl) ⟶ (MgCl2) + (H2).
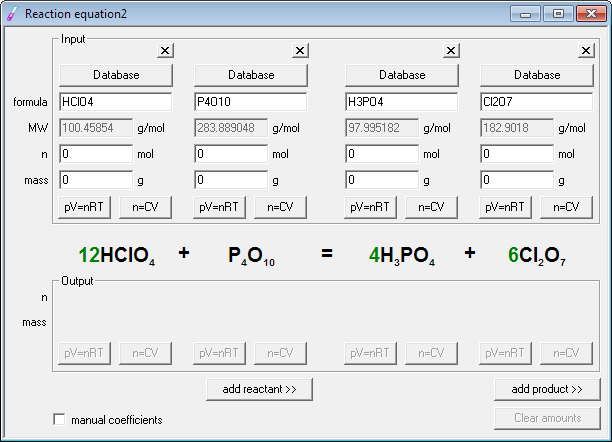
The equation (Mg) + (HCl) ⟶ (MgCl2) + (H2) is clearly unbalanced because on one side, there is only 1 hydrogen atom, but on the other side, there are 2 (also unbalanced Chlorine, but they both come from the same compound so this becomes really simple to balance). you need to make sure (because of the law of conservation of matter-matter cannot be destroyed or created) that if you have a definite amount of something on one side, you have an equal amount of it on the other side. So, balancing an equation is just like balancing the x in an algebraic equation. You can read my explanation below to get an idea for this, but basically, the coefficient is just telling the chemist how much of a specific atom, molecule or compound it takes to gain the desired product. It gives us a way to measure a reaction and use stoichiometry to gain the exact amounts desired of a specific product. The coefficient in a balanced equation is an idea the concept of telling the chemist that if the atoms, molecules and compounds are balanced, there are balanced amounts of the atoms, molecules and compounds on the other side in the product.


 0 kommentar(er)
0 kommentar(er)
